Expandable Flake Graphite Intercalation: Expandable flake graphite, also known as intumescent flake graphite, or simply “expandable flake”, is a form of intercalated graphite. Intercalation is a process whereby an intercallant material is inserted between the graphene layers of a graphite crystal or particle. After intercalation the resulting graphite material takes on new properties that are a function of the intercallant and the way it associates with the host (graphite) species. Both physical and chemical properties, including crystallographic structure, surface area, density, electronic properties, intumescent behavior, chemical reactivity, etc., may be affected by the intercallant.
A wide variety of chemical species have been used to intercalate graphite materials. These include halogens, alkali metals, sulfate, nitrate, various organic acids, aluminum chloride, ferric chloride, other metal halides, arsenic sulfide, thallium sulfide, etc. The primary type of graphite intercalation compound described in this article is the “sulfate” intercalation compound sometimes referred to as “graphite bisulfate”. This material is manufactured by treating highly crystalline natural flake graphite with a mixture of sulfuric acid and certain other oxidizing agents which aid in “catalysis” of the sulfate intercalation. The resultant product is a highly intumescent form of graphite that has proven useful in applications that include fire retardants, high performance gaskets, conductive fillers, electromagnetic pulse and radiation shielding, foundry products, and many others.
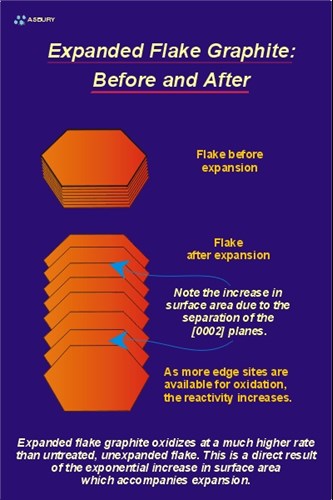
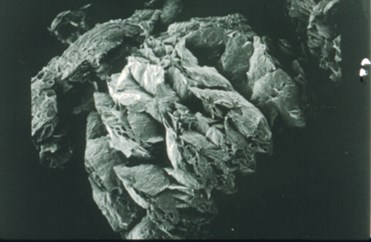
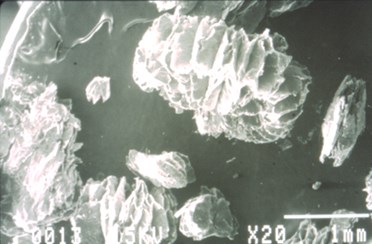
The primary reason for “bisulfate” intercalation is to impart the ability of the treated flake graphite to intumesce, exfoliate, or expand when heated. Exfoliation is a true volumetric expansion resulting from the crystallographic de-lamination that occurs parallel with the “c” crystallographic axis of the graphite flake. The figure, below-left, is a schematic of what occurs during expansion. The top illustration shows a single piece of flake graphite, which has been intercalated but not exfoliated. The bottom illustration is the same flake of graphite after being exposed to a temperature high enough to affect expansion/exfoliation. High temperature causes the expansion agent to gasify, producing enough pressure to push adjacent graphite layers apart. The scanning electron micrographs, below, show an actual graphite flake before and after expansion/exfoliation.

Flake Graphite, Unexpanded
Intercalated Flake Graphite, Expanded
Note the accordion-like morphology that results from the flake’s expansion parallel to the “C” crystallographic axis.
Exfoliation of the flake graphite results in an overall decrease in bulk density, and an approximately 10 fold increase in surface area. Typically the increased surface area results in a material that has increased chemical reactivity. This reactivity is especially apparent in oxidation rate of uncompressed material.
Exfoliation - The Mechanism: The actual cause of expansion/exfoliation is the increase in volume, and resultant pressure, caused by the rapid heating of the intercallant. A simplified way to view the process is to model the intercallant as a liquid or solid phase that is fixed between the graphene layers. Heating of the treated graphite results in conversion of the intercallant, from a liquid or solid phase, to a gas phase. Gas formation results in an increase in volume of the intercallant of approximately 1000 fold. The pressure generated by this volume increase forces the adjacent graphene layers to separate, resulting in the accordion expansion observed.
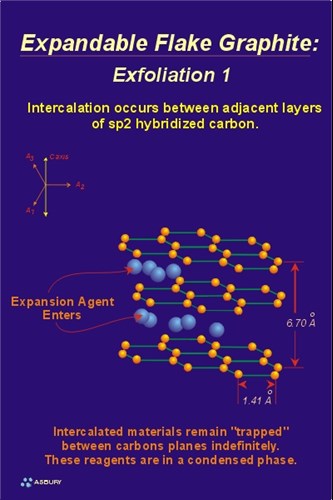
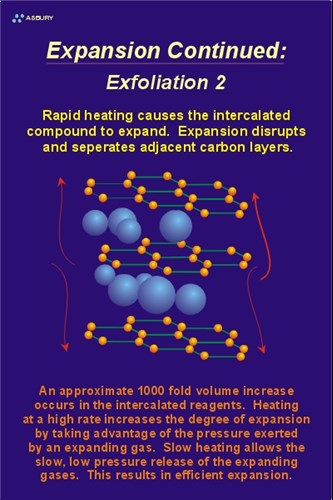
The two Figures, left and right, illustrate schematically the process of expansion / exfoliation. The Figure on the left shows the expansion agent (blue spheres) in their metastable residence between adjacent graphene layers. The Figure on the right shows the expanded intercallant and the results of the expansion on the graphene layer spacing. Note that the interlayer dimensions, shown on the left are equal to approximately 3.35 Å (6.7 Å /2). The interlayer spacing, post exfoliation, is some value greater than 3.35 Å . An increase in graphene layer spacing is a result of the process.
Definition of the Expansion/Exfoliation Ratio: Asbury Graphite’s definition of expansion ratio is the quotient of expandable graphite’s post heating expansion volume and original sample mass. The “units” are reported simply as “expansion ratio”, “exfoliation ratio”, or “heat expansion”. If one performs a dimensional analysis on the expansion quotient, the units are volume/mass, which reads like a specific volume. Due to certain complications such as volatile content the “expansion ratio” is not a specific volume. Depending on the size of the test crucible, the test method requires that either one gram or 0.5 gram of intercalated graphite be expanded and the final expanded volume measured. Although the true density of expandable graphite is approximately 2.2 g/cm3 the bulk density is lower. The result is that a cubic centimeter of expandable flake graphite is close enough to 1 gram in mass to assume that the expansion ratio is the measure of the volume change realized when approximately one cubic center of intumescent flake graphite is exfoliated. The values reported for “heat expansion” are much easier to visualize when making this assumption.
Optimization of Expansion/Exfoliation Ratio: Not all bisulfate intercalated graphite expands equally. Differences in treatment type, particle size, etc., all have a bearing on the overall expansion of the product. Some applications that utilize expandable flake graphite require high expansion ratio, some applications require a low expansion ratio. In the following sections some of the factors that affect the magnitude of the expansion ratio will be discussed.
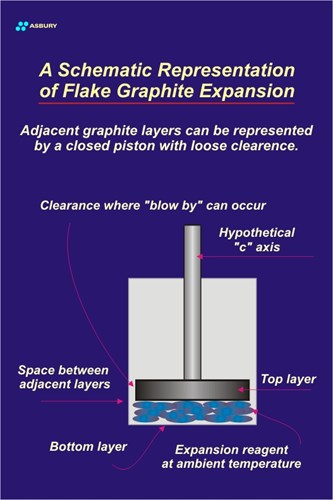
Expansion Vs Temperature: Most flake graphite exfoliation is affected by subjecting the intercalated product to heat energy. As explained above, heating of the intercallant results in a phase change that provides the inter-laminar pressure required to force layer planes to separate. The maximum expansion potential of a specific grade of intercalated graphite can only be realized if the heating rate used to affect exfoliation is rapid. Slow heated or heating in “ramping” stages will typically produce low expansion and can even result in no expansion. Since the expansion process depends on harnessing the force developed by an expanding gas, it is important to develop maximum force by causing a sudden, rapid gas expansion. To do otherwise, i.e., slow heating and therefore slow expansion, will result in less than the threshold gas pressure required to affect expansion. A good way to view the above concept is to model the flake graphite exfoliation process as the expansion of a gas in a cylinder with a loose fitting piston.
The Figure below-left illustrates this model. Assume that the bottom of the piston and the bottom of the cylinder represent two adjacent graphene layers. Also assume that the clearance between the cylinder walls and piston represent the prismatic edges that define each graphene layer. Ultimately gas will escape through these edges. The blue spheres trapped between the piston and cylinder bottom represent the intercallant substance assumed to be bisulfate in this case. Let’s slowly heat the system that consists of the cylinder/piston and its contents.
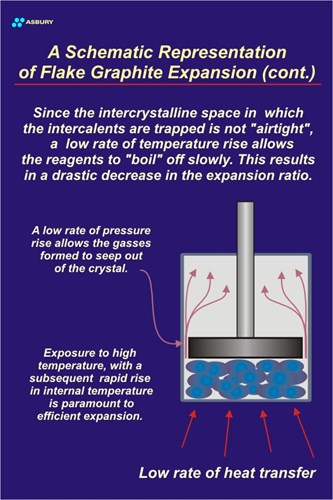
The Figure to right illustrates this slow heating process. Since the heating rate is low, the pressure rise is corresponding low. This is because gases are produced slowly and have time to “percolate” out of the system before pressure builds to a high enough level to cause delamination. The resultant material has not expanded to its full potential.
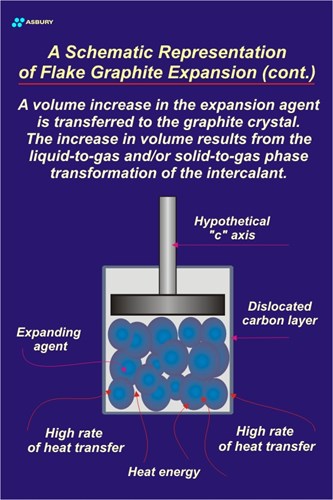
Let’s take the same system and apply heat at a much faster rate. The Figure to the left illustrates this scenario. When heat flow is high the gases produced by the vaporization of the intercallant do not have time to percolate out of the cylinder-piston clearance. The result is high pressure and the resultant force required to lift the piston, or exfoliate the graphite crystal, is realized.
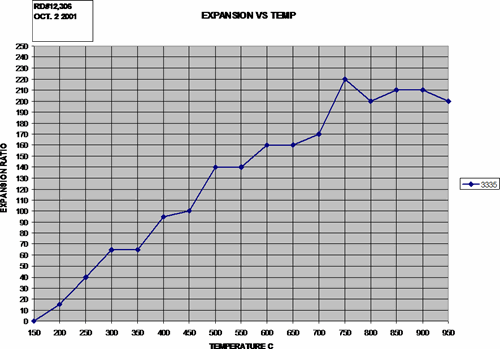
The graph below is a plot of expansion ratio vs. heat treatment temperature for a +50 mesh (<300 micrometers) expandable flake graphite. The plotted data clearly shows the strong relationship of high temperature to high expansion. Note that the maximum expansion is at about 700 ºC and higher.
Expansion vs. Particle Size: The second, and equally important, material parameter that affects the expansion ratio is the particle size of the intercalated flake graphite. In general, particle size is directly proportional to expansion ratio. Large flakes typically have higher expansion ratios than smaller flakes. This phenomenon can be understood by looking at another simple model. Let’s assume that a graphite flake can be modeled as a disc. This is a reasonable assumption since flake graphite is somewhat hexagonal in outline.
The figure below illustrates flake graphite’s discotic morphology. Although not to scale, the value of “h” shown below represents the interlayer or graphene layer spacing of the crystal. This space is where any vaporized intercalated species would exit the crystal. Discounting any defects or variation in interlayer spacing due to the insertion of the intercallant, the value of “h” is constant at 3.35 Å.
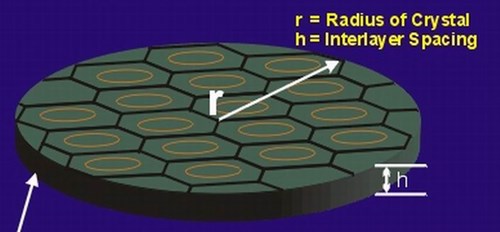
As indicated in the above figure, “r” is the radius of the disc element illustrated. The total area available for gas escape from a single layer is equal to: 2prh. The volume from which intercallant gas is produced is equal to: pr2h. If one looks at the ratio of edge area to disc volume, 2prh /pr2h, it can be seen that the ratio varies as 2/r. You can see that as the radius decreases the ratio 2/r gets very large.
The Figure below presents a graphical representation of the (edge area/disc volume) vs. particle radius.
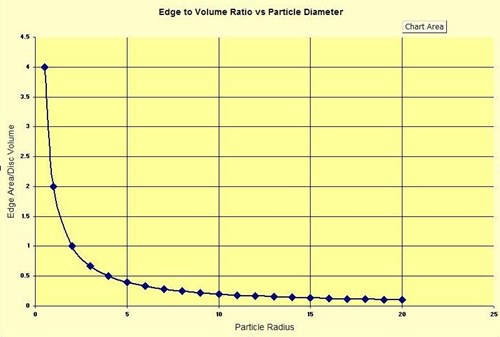
As illustrated by the plotted values, the smaller the particle radius the higher the value of the edge area/disc volume ratio, and the larger the particle radius the smaller the edge area/disc volume ratio. As the particles get smaller intercallant gases have a more efficient escape pathway. Rapid gas escape prevents development of the pressure required to push adjacent graphene layers apart. The result: low expansion ratio. In addition to the “pressure” effect modeled above, the thickness of the graphite flakes also has a significant effect on expansion ratio. Since the interlayer spacing is the same regardless of the flake thickness, thicker flake will expand proportionally more than thinner flakes. When grinding, screening, or otherwise reducing the particle size of flake graphite, cleavage parallel to the basal plane (basal pinacoid) occurs. The result is the creation of thinner flakes with subsequent reduction of the expansion potential of those flakes. The effect of particle size on graphite exfoliation has good empirical support.
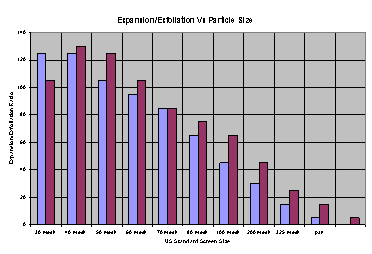
The Figure above presents a graph of Particle Size vs. Expansion Ratio.
This data was generated by screen fractionation of a sample of commercially available intercalated graphite. The various fractions were exposed to a furnace temperature of 950 ºC to affect exfoliation and the volume of the expanded flake was compared. Note that the same sample mass was used for each test (Asbury test method E4-4). The Graph shows a clear trend in the data; as particle size decreases the exfoliation volume decreases accordingly. Formulators who must chose the correct expansion potential for a specific application can use this property variable to their advantage. Specific expansion ratios can be adjusted by mixing different grades of differing expansion potential to control the ultimate expansion ratio of the finished product.
Asbury is a major world wide supplier of expandable flake graphite. Numerous grades are available, which vary in particle size distribution, carbon content, pH, and expansion ratio. In addition to the 10 standard products listed in this Web site we have the ability to provide customers with tailor made materials designed to precisely fit the application at hand.
For technical inquiries about expandable graphite please feel free to contact the Asbury Technical Services Department at [email protected].
For additional information on Expandable Graphite, please read the following Heat Aging and Chemical Aging studies:

