Graphite is composed of layers of carbon atoms that are arranged in 6-membered, hexagonal rings. These rings are attached to one another on their edges. Layers of fused rings can be modeled as an infinite series of fused benzene rings (without the hydrogen atoms). Carbon atoms in these ring arrays are in the sp2-hybridized state. In the sp2 molecular orbital model each carbon atom is attached to three other species, three other carbon atoms in the case of graphite. In this bonding mode the bond angle between adjacent carbon atoms is 120. These “ring arrays” are arranged in large sheets of carbon atoms, and individual sheets are known as graphene layers. The carbon-carbon bond length in a layer plane is 1.418. Graphene layers are stacked one on top of another parallel with the “C” crystallographic axis of the hexagonal 4-axis system in which graphite crystallizes.
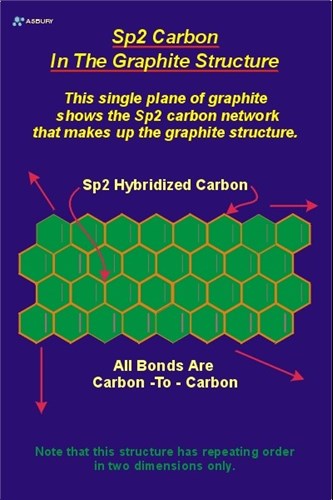
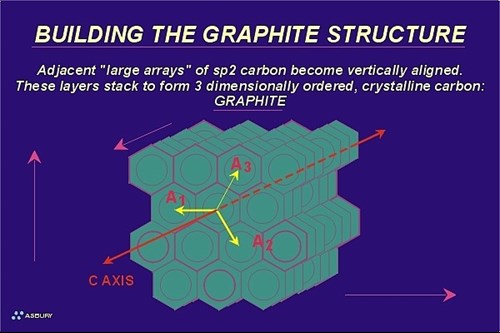
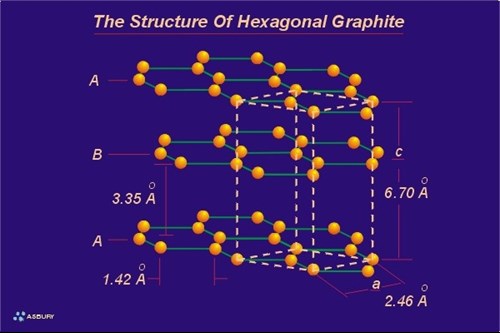
Graphene layers are precisely “indexed” so that an exact three-dimensional repeating order is developed and maintained throughout the crystal (as is required for any crystalline solid). In this arrangement each graphene layer is indexed to the layer above and the layer below in such a way as to form a repeating alignment every second layer. In other words, the carbon atoms in the first layer indexed are located in precise alignment with the carbon atoms of the third layer indexed. Correspondingly, the carbon atoms of the second graphene layer are in alignment with the atoms of the forth layer. This configuration is known as an “ABABAB” structure. All the “A” layers are aligned with other “A” layers, and all the “B” layers are aligned with other “B” layers. A simplified view of this model is to imagine the “A” layer fixed in space. Then align the “B” layer so that directly below the center of each “A” layer hexagon there is located, dead center a carbon atom in the “B” layer.
Since graphite is a crystalline solid it can be defined by a basic unit cell. Graphite is hexagonal and therefore is defined by a hexagonal unit cell. The hexagonal unit cell of graphite is a rhombic prism (not a hexagonal prism). The prism is defined by six surfaces: four sides and a top and bottom. This cell encloses or partially encloses the carbon atoms from three graphene layers, so one could say the unit cell starts at layer “A1” and finishes at layer “A2.” Layer “B1” would then be located within the unit cell. Each unit cell contains a total of four carbon atoms (z=4). One and one sixth (1 1/6) atoms each comes from the first and third layer of the unit cell (top and bottom) and one and two thirds (1 2/3) atoms comes from the second layer (middle layer). Since each unit cell contains “centered” and “edge” carbon atoms the unit cell is considered a “centered” unit cell as opposed to a primitive unit cell. The unit cell dimensions are the lengths of the imaginary line connecting in-plane carbon atoms (a) along cell edges that are parallel with the graphite layers, and the length of the imaginary line connecting between-plane carbon atoms (c) between the first and third layer (the edge perpendicular to the graphene layer). These dimensions are: a= 2.456, c= 6.694. Since the distance between two layers (c) is 6.694, the distance between any two adjacent layers is 6.692 /2 = 3.346 (c/2).
Carbon atoms in the graphite crystal are in the sp2-hybridized state. As discussed in the section on organic chemistry this means that carbon atoms support two bonding components a sigma (σ) component, and pi (π) component.
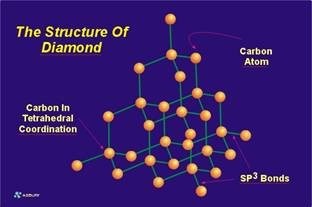
The sigma bonds are “hard” bonds and are similar in character to the pure sp3-type sigma bonds, which form the 3 dimensional giant molecular structure of diamond. The sigma bonding system acting within a single graphene layer is exceedingly strong. In fact, it is probably the strongest 2 dimensional network structure known, and is the structural backbone of all carbon/graphite fibers, and carbon nano-tubes. The strength of the sigma bond in carbon is also illustrated by the high hardness of diamond. Each carbon atom within the graphene layer has three of these sigma atomic orbitals, which combine with similar orbitals of adjacent carbon atoms forming the molecular bonds that hold the layer together (see sp2 bonding in the organic chemistry section). In contrast, the carbon atoms of the diamond structure each have 4 such bonds. From an atomic perspective, one could “walk” from one carbon atom, to any carbon atom within a graphene layer using the “hard” sigma bonds as bridges to traverse the pathway between carbon atoms. However, your stroll would be limited only to the two dimensions covered by the graphene layer you were on. In diamond, the entire diamond molecule can be traversed, in a similar fashion, in any direction, up, down, or side-to-side so that any carbon atom can be reached from any other carbon atom using “sigma bond bridges” to traverse the path.
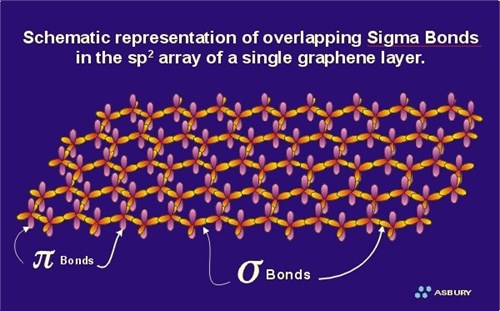
The stiff sigma bonds that give rigidity and high tensile strength to a graphene layer do not provide any support in the perpendicular direction. In other words they do nothing to hold the graphite crystal together in the “c” direction. Graphene layers are real structures and require some force to maintain sheet alignment as well as the proper equilibrium separation distance. The function of crystal support in the third dimension, the “c” dimension, is supplied by the second bonding component of the sp2 model known as the pi component or pi bond.
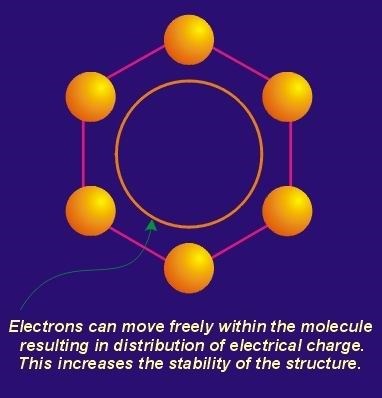
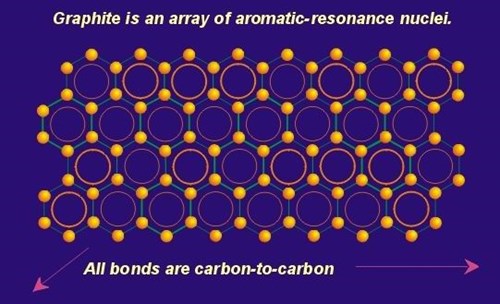
The pi component is the result of weak, secondary electrical bonds formed by the overlapping pi (p) orbitals of the sp2 carbon network within each graphene sheet. Each carbon atom has one pi electron. This electron has a high probability of being found in a region just above or below the plane formed by the carbon atoms in a graphene layer. Since each graphene layer can be viewed as a system of fused six-carbon rings, one can also imagine that superimposed above and below each ring there exists a ring or donut shaped region, containing six pi electrons (one from each carbon atom). These pi electrons are not stationary, but are “de-localized” within the confines of the donut shaped region they occupy. This de-localization of pi electrons results in what is known as resonance energy or resonance stability (See section on Aromaticity and Resonance). The stability here is chemical stability, which is spread throughout the entire graphite structure. In non-quantum terms, the effect is one of “spreading” out the electrical charge across the entire array of carbon atoms. In addition to the delocalization of the pi orbital electrons within the region located immediately above a given ring, this delocalization is “conjugated” throughout the contiguous graphene layer. This means that there is some mechanism for pi electrons to be delocalized not only in their immediate orbital, but to move back and forth between and within adjacent orbitals.
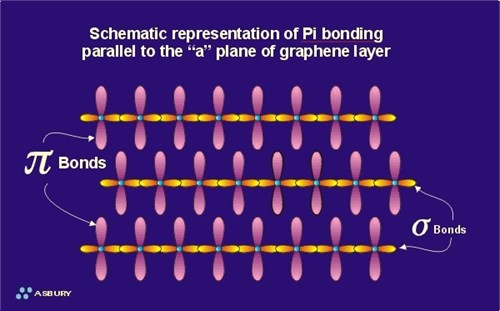
As mentioned above, the pi system in graphite does not provide significant in-plane structural support. However, each pi system produces a “force field” which “communicates” with the field generated by the graphene layers located above and below it. These interacting fields provide the weak, secondary covalent bonding system, which hold adjacent graphene layers in their equilibrium position of 3.346 of separation parallel to the “c” axis. However since these forces are truly weak, adjacent graphene layers are easily peeled away from one another, or repositioned horizontally relative to one another. The ability of graphite to form a lubricious film is the result of this “break-away” layer structure.

