That is a somewhat specific definition that restricts the study to “the fate of organics” and eliminates carbon from “inorganic” sources, such as carbonatites, etc, from this branch of geology. As noted elsewhere in these articles many types of natural graphite, such as flake and amorphous, are formed from the action of geologic energy on organic based deposits. Therefore the study of organic geochemistry is very important in understanding the complete “graphite cycle.” Note that graphite formed from “other” carbon reservoirs considered “inorganic” are not included in this discipline or in this article.
The Carbon Cycle
Carbon is probably the element whose “cycle” is easiest to follow through the geosphere: Consider a volume of carbon dioxide gas in the atmosphere. Green plants take up this carbon dioxide and through the chemical action of photosynthesis “fix” it into solid carbohydrate materials that supply energy and mechanical support for the plant tissues. After death the fixed carbon in the plant may be released, once again as carbon dioxide, as the tissues are fed on by saprophytic organisms. In this “atmospheric” cycle the carbon never enters the earths crust. This is one possible scenario for the cycling of carbon.
In addition to the “atmospheric carbon cycle” described above, another possibility exists whereby dead plant tissues “fall” into a zone where they are protected from oxidation and the saprophytes that live in such oxygen rich environments. If this second scenario occurs then it is possible that over many years the plant tissues will accumulate into layers or lenses of “carbohydrate” that are protected from the prevailing oxidative degradation prevalent on or near the surface. The isolation of the carbohydrate-rich lens is achieved as a result of burial by sand, silt, carbonate tests or other blanket of geologic material. Once burial occurs the plant tissues are “temporarily” (although this can be many years) isolated from any future potential to be degraded by surface phenomena.
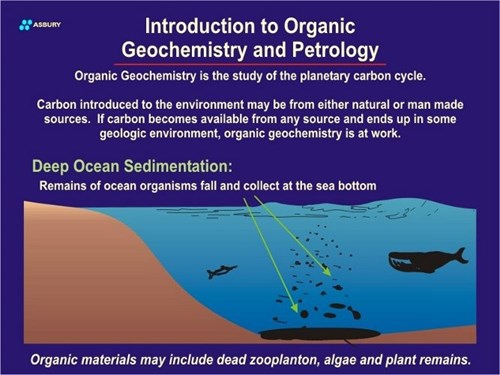
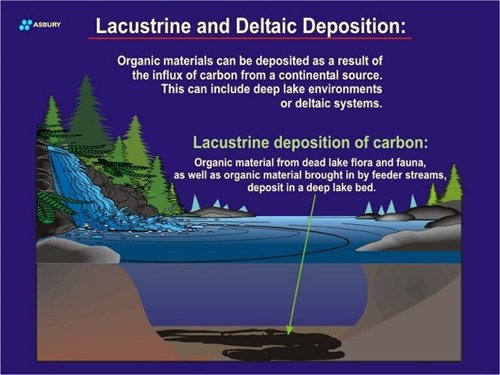
The above paragraphs propose a possible pathway for carbon formed by plants (or animals) located on land. The same scenario also exists for organisms that live and die in oceans or deep lakes. In the case of aquatic species carbon is fixed by plants (algae, etc.) in a manner similar to terrestrial species.
After dying these organisms sink to the seafloor or lakebed. If deposition is fast and bottom conditions anoxic (reducing) solid carbon will remain and build up as it does on land. Subsequent burial of the carbon lens leads to the same isolation and protection as in land based deposits.
Burial of terrestrial, lake bed, deltaic, or ocean bottom organic carbon may continue until the lens is deep within the earth’s crust. As the depth of the lens increases the heat and pressure work that it is subjected to increases. The total amount of work delivered to a given carbonaceous lens is what determines whether it will remain as amorphous carbon or be converted into crystalline graphite.
Fossil Carbon and Fossil Molecules
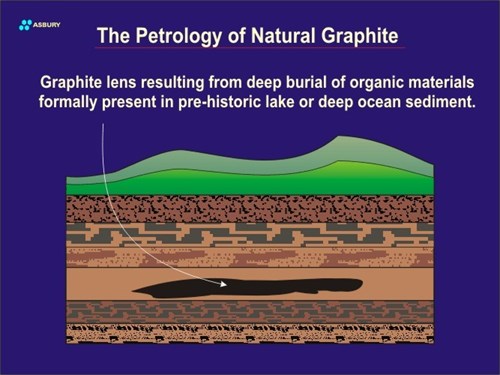
In non-graphite-forming deposits, the identity of the original deposit can have a significant bearing on whether the ultimate form of fossil carbon is coal, oil, gas, Gilsonite, etc. Not all types of carbon will yield the same type of fossil carbon. Fossilized carbon can in some cases be traced to specific sources of origin. In other words, there are fossil materials present that retain some identity of their organic source material (biomarkers). For example, careful study of crude petroleum will, in some cases, show the presence of chlorophyll related structures that give some clue to its original organic source as green plants. These structures can actually be thought of as “molecular fossils.” Another example of the “traceability” of fossil carbon to its source material is clearly seen in low grade coals. Microscope examination of these coals shows the presence of fractions (macerals) containing the remains of primordial plant tissue. These macerals show cellular structures as well as macroscopic plant structures quite clearly. In some cases it is possible to identify with some specificity the types of plants that form a given coal seam.
In environments that are potentially graphite producing, including those containing anthracite coal, the chemical identity of all types of carbonaceous deposits tend to converge as the level of energy to which they are exposed increases. In other words, whether the deposit is land based vascular-plant-derived or ocean based algal-derived the resultant natural graphite will form from a proto-graphitic carbon that is thermally matured to the point where its original organic source materials are not discernable or are discernable only with great difficulty.
Kerogen, the building block of some natural graphite
Before proceeding with a closer look at the formation of graphite from organic based carbons, it should once again be stated that natural graphite can also form from inorganic carbon. However it is likely that much of the commercially available flake graphite and all amorphous graphite are formed from organic precursors.
Regardless of the source biogenic material all geologic organic residues are “condensed” during deposition and consolidation. The result of this condensation is a naturally occurring polymer material known generically as kerogen. Kerogen is a high molecular weight material composed primarily of carbon, hydrogen, oxygen, nitrogen, and sulfur. Various other elements, including trace metals such as magnesium may also be present in the “molecule.” Examination of the various structures that make up a kerogen “unit” show the presence of alkane chains, cyclohexane chairs, saccaride units, porphyrin structures, aromatic ring structures, cyclic alkanes, and others all linked together rather randomly.
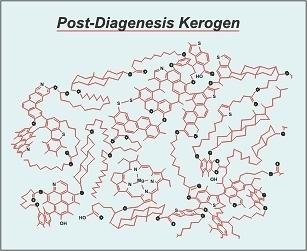
(image: After Behar and Vanderbrouck 1987)
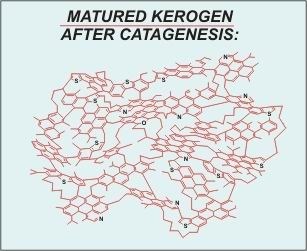
(image: After Behar and Vanderbrouck 1987)
“New” kerogen is kerogen that has formed during the first stage of sedimentation and consolidation known as diagenesis. At this early state of its geologic history kerogen is high in volatile elements such as hydrogen, oxygen, and nitrogen. It is also rich in the less thermally stable organic groups such as alkanes, hydroxyl groups, ether linkages, etc.
“Matured” kerogen is kerogen that has gone through deposition and consolidation (diagenesis) along with subsequent exposure to increased temperature and pressure. During this process the kerogen is subjected to heat-and-pressure work. The result is condensation of the overall structure. Less stable groups and species are “cooked-out” of the polymer resulting in a significant increase in the amount carbon compared to all other species present.
Another very significant change (important regarding graphite formation) is the increase in the aromaticity of the overall structure. “Benzene ring” structures are formed from the condensation of other organic groups and these structures are squeezed together, along with existing benzene rings, to form polynuclear aromatic units.
The presence of “aromatic” (see sections on “Organic Chemistry” and “Aromaticity and Resonance”) carbon is very important since graphite is built up of aromatic structural units. The presence of these units is prerequisite to the formation of crystalline carbon. Once the primordial source carbon is thermally matured, aromatic units will not form.
Diagenesis, catagenesis, and metagenesis
Organic geochemists have created a system of terms, diagenesis, catagenesis, and metagenesis, which are used to describe the various steps in the maturation of organically derived carbons from their organic precursor sediments, through the formation of various fossil carbons, and finally graphite.
In general the development of one type of carbon at the expense of another is a strong function of temperature and pressure. Although not all carbon will be converted to graphite at high temperature and pressure, these conditions must always be part of the conversion process. Note that not all carbon passes through all three stages. Maturation may be terminated at any point in any stage depending on the “local” conditions.
Diagenesis, mentioned earlier in this article, is the primary stage of deposition and involves the processes prior to and up to the early stages of burial. This includes deposition processes that occur under conditions of low temperature and pressure. Both biologically as well as chemically induced changes occur during this process. The diagenesis stage also includes compaction and consolidation but not complete consolidation into rock, a process known as lithification. The identity of the carbon during the digenetic process is still organic with many of the constituent organic molecules still remaining, although in condensed form. Diagenesis is a dynamic process in which the sediments are attempting to reach equilibrium with the constantly changing geologic environment. Important parts of this change are reactions with, and expelling of, pore water which is squeezed out of sediments as burial pressure increases. Diagenesis is believed to occur at temperatures below 50 C. The onset and completion of diagenesis are hard to define, however, as temperature and pressure increase the identity of constituent organic molecules change as a result, diagenesis ends, and “stage II” catagenesis ensues (Killops 1993).
Catagenesis is the second stage of maturation of organic carbon on the path to becoming graphitic. This geologic process accounts for very significant changes in the biogenic materials that make up the carbonaceous sediment. During catagenesis the temperature increases, the pressure increases, and both organic and inorganic constituents “adjust” their phase or form to compensate. The process of “lithification” begins during this stage. Generally speaking a rise in temperature results in the volatization of unstable species or elements that are weakly attached to carbon atoms. Increased temperature and pressure also result in the cessation of biogenic processes. One way to express these changes is to look at the ratio of oxygen to carbon, or hydrogen to carbon as the sediment matures. In almost all cases as biogenic material matures in a geologic environment the volatile elements such as oxygen and hydrogen are significantly reduced, resulting in a reduction in the O/C and H/C ratios. A typical O/C ratio value for a fully matured, catagenesis stage carbon might be less than 0.1. This means that for every 100 carbon atoms there are less than 10 oxygen atoms. Similar reductions in the level of hydrogen are also apparent.
An oversimplified, but nevertheless illustrative explanation of what occurs in carbonaceous materials during catagenesis is to look at the process as “a relaxation process.” In this case the “relaxation” is the structural changes taking place in the kerogen as it adjusts to the higher temperature and pressure conditions realized during the catagenesis stage. As higher temperatures distill and condense the structure, high pressure works to adjust the “allowed volume” available to the “distilled residue.” Unless carbonaceous materials are subjected to the elevated temperatures and pressures of catagenesis the kerogen structure will remain relatively unchanged and the conversion to graphite will not occur.
The third and final stage of kerogen maturation occurs during metagenesis. Metagenesis is the stage where classic metamorphism occurs. As more and more sediment forms, the material deposited above compresses that already deposited below. Sedimentation and compaction may continue until the carbon-bearing layer, originally at the ocean floor (if it’s a seabed deposit), is 10-40 kilometers below the surface of the crust. Subduction of the carbon lens to similar crustal depth may also result from tectonic forces. At the range of burial depths indicated the pressure and temperature may vary between perhaps 4-14 Kbar and 750-1000 C (Granulite facies metamorphism).
Exposure to the extremes of metamorphism causes the kerogen to release any remaining volatile components. Methane may be evolved, sulfur is lost as H2S, and all “lower boiling” components including aliphatics and carbonyl oxygen (C=O) are lost. The predominant form of carbon remaining is contained in aromatic ring structures (Killops 1993). Of coarse the ultimate pressure and temperature of the enclosing crust takes tens if not hundreds of millions of years to be reached. Over this significant period of time the carbon lens is manipulated by the crust and the carbon goes through various states of “development” on its way to being converted to graphite.
Formation of the graphite crystalline phase from amorphous carbon
Now that the basics of the carbon/graphite cycle have been described a hypothetical example of the geological synthesis of a “graphite unit” will be presented. Our goal here is to provide only a very basic “schematic” that conveys the idea’s of the solid-solid phase transformation of amorphous carbon to crystalline graphite. The reader should be reminded again that the time interval over which this transformation occurs is on the order of 10(7)years!
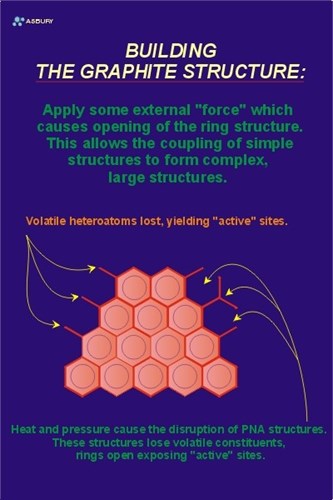
Let’s start this schematic solid/solid phase transformation by placing a volume element of “kerogen” into the upper earth’s crust. The figure to the left shows a relativity simple polynuclear aromatic structure to be used as a model-kerogen for the example. Assume that the spherical groups attached to the hexagon edges represent volatile or otherwise unstable species that are part of the post diagenesis kerogen.
The next step is to perform heat and pressure work on the kerogen volume element. After some time in “catagenesis” the kerogen “adjusts” to the local environment by losing most of its volatile components and forms an “activated” intermediate species. The Figure to the right is a model of such an “active” material.

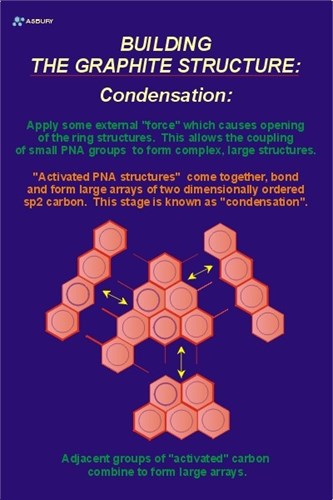
Due to the close proximity of the other volume elements of activated kerogen in this hypothetical “catagenetic” environment there are reactions that occur which are the result of reaction between kerogen molecular sites that have been stripped of their volatile species or have been made otherwise reactive due to the local environment. As explained above the reactions tend toward the development of solid carbon in aromatic structures at the expense of aliphatic and other unstable structures. As more active sites become available, adjacent kerogen groups react with one another. Smaller units of carbon combine to form larger units in a process termed “condensation.” The Figure to the left is schematic representation of the condensation of small polynuclear aromatic units. These units combine and grow to form structures composed of large arrays of aromatic carbon.
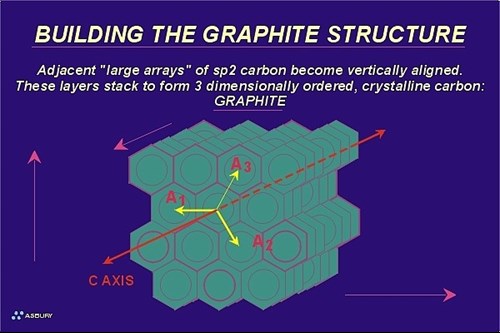
The Figure on the right illustrates these large arrays. The yellow arrows illustrate the two-dimensional growth direction. At this point in the natural graphitization process the carbon is still in the amorphous phase with long range order occurring in only two crystallographic directions.
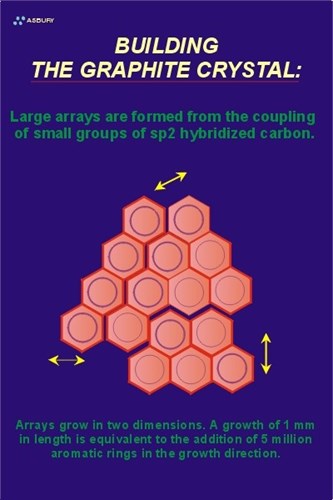
In order for carbon to be considered graphite, it must meet certain crystallographic criteria that include the precise spacing of carbon atoms within a graphene layer as well as precise spacing of the layers themselves. Two environmental factors contribute to the overall driving force of this phase transformation: pressure and temperature. Amorphous carbon is bulky so as pressure increases reactions that tend toward higher density follow. Going from a low density arrangement of atoms to a more compact, high density configuration requires motion, the increased heat of a the metamorphic environment provides the kinetic energy to carbon atoms that helps them “relocate” into a hexagonal lattice.
The metagenesis phase ends with the formation of crystalline graphitic carbon. The entire process leading up to metagenesis can be viewed as a solid-solid phase change that starts with low density, disordered carbon and ends with the formation of high density, ordered carbon. The Figure below presents this concept schematically:
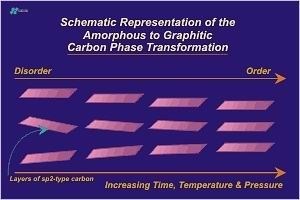
At this point carbon atoms are arranged in a hexagonal lattice. (See section on the hexagonal crystal system.) Atoms within a graphene layer are located 1.42 Angstroms from their nearest neighbor carbon atoms. Graphite layers are 3.35 Angstroms from adjacent layers. Virtually all of the carbon atoms, except edge atoms, are aromatic (sp2 hybridized) and the structure as a whole is stable up to temperatures above 3500 C. Crustal conditions never approach such temperature extremes so for all practical purposes the prograde metamorphism of carbon ends here. Retrograde metamorphism (backwards metamorphism) will not occur. However if the environment becomes oxidizing graphite may react accordingly.
The Figure below shows the final graphitic carbon resulting from the conversion of amorphous organic- based carbon to crystalline inorganic carbon. Readers who would like to learn more about organic petrology are urged to read the excellent book “An Introduction to Organic Geochemistry” by Stephen and Vanessa Killops, 1993, Longman Scientific & Technical Publishing, Essex, England.