To begin, let’s go back to the root of Organic Chemistry. Although graphite is considered an inorganicmaterial, its “roots” are firmly in organic chemistry – or loosely speaking - the study of carbon compounds. This definition can be further refined to include only carbon compounds derived from “organic” materials such as plants, animals, etc. One would expect then that graphite should be considered “organic” since many types of natural graphite are formed from what began as truly organic carbons, and 99% of synthetic graphites are derived from petroleum or other organic carbon sources. However, graphite is an element and not a compound, and graphite is typically considered a mineral (by definition a mineral cannot be organic), so an argument can be made for its inorganic nature.
The element carbon is unique in that it probably forms more compounds than all of the other elements combined. This compound-forming ability is the result of the structural flexibility of the carbon bond. If you were to review the structural formulas of a number of carbon compounds you would find that carbon bonds to itself and other species in ways that result in the formation of molecules composed of short chains, long chains, small rings, large rings, “crowns,” geodesic spheres, combinations of rings, combinations of chains, and combinations of all the other combinations! This ability is the result of the multiple bond types that carbon can form.
The Three Bonding Schemes of Carbon
Depending upon the chemical environment a carbon atom can form what chemists refer to as single, “double,” or “triple” bonds with adjacent carbon atoms or with “heteroatoms.” The terms “double” and “triple” are quoted because in reality a “double” bond is not simply two single bonds and a “triple” bond is not simply three single bonds.
Each of these bonding schemes is the result of a phenomenon know as “bond hybridization.” The term hybridization is used because the four bonding electrons found in the bonding orbital of carbon are not “fixed” in their ability to combine with other atoms. Instead, these electrons can adjust their “orbital structure” to allow the formation of bonding schemes that fit the chemical environment at hand. Recall that the electronic structure of the carbon atom is 1s2 , 2s2, 2p2. The bonding electrons being referred to are the two electrons in the 2s suborbital and the two electrons in the 2p suborbital (total of 4). These are the four bonding electrons that take part in all chemical reactions or bonding interactions that carbon atoms are involved in.
As an example of bond hybridization consider that carbon can combine with the element hydrogen in various ways. Below, three different compounds of carbon and hydrogen will be described. In each of these compounds it will be shown that carbon utilizes its bonding electrons in three different ways. The reader should keep in mind that the schemes discussed are not limited to compounds containing only carbon combined with hydrogen. Also, the perspective presented is a non-quantum, qualitative perspective designed to provide the reader with a basic “nuts and bolts” understanding of carbon.
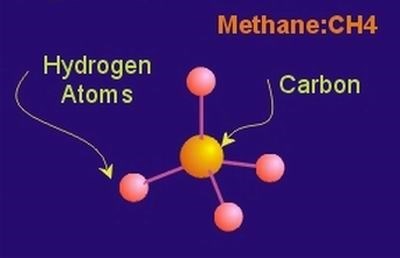
Sp3 Bond Hybridization
Under certain conditions, a single carbon atom can combine with 4 hydrogen atoms. In this particular compound, carbon has four bonds, one to each of four hydrogen atoms. The compound, a gas, is called methane, CH4. Methane is a major component of natural gas. In this bonding scheme the bonding electrons in the 2s and 2p orbitals are said to be sp3 hybridized. In this situation, each of the 4 bonding electrons contributes in the formation of a “hard” electron/electron bond between the carbon atom and the specific hydrogen atom with which it is associated. The bonds are all equivalent and the bonding electrons are, more or less, “pinned” between the carbon and hydrogen nucleus. In order to minimize repulsive forces, the four bonds to hydrogen are arranged tetrahedrally with bond angles of 109.5 deg. This type of hard bond is known as a sigma bond. To review: carbon in methane is sp3hybridized, with four sigma bonds, one to each of four hydrogen atoms.
Sp2 Hybridization
If we put the same carbon and hydrogen atoms in a different chemical environment, the possibility exists for another combination arrangement. In this new scheme two carbon atoms combine with each other and with 2 hydrogen atoms each. In other words each carbon atom is bonded to one other carbon and two hydrogen atoms. The compound is ethene, C2H4(ethylene). The type of bond in this case is known as sp2 hybridized. Note that in this new type of compound each carbon atom is attached to only three other species, as opposed to the case above (sp3) where each carbon was attached to four other species. In ethene each carbon atom has three hard electron/electron bonds, one each to the adjacent carbon and two hydrogen atoms. Sp2 hybridization is important to solid-carbon since it is the primary type of carbon present in solid black carbon materials.
The obvious question regarding carbon bonded to three other species, with four bonding electrons, is what happens to the fourth bonding electron? If one refers to the literature for an illustration of the molecular structure of ethene it will be seen that the fourth bonding electron (unaccounted for in terms of hard sigma bonds) is shown as a double bond between carbon atoms.
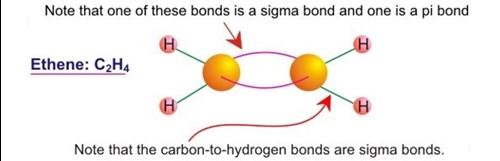
The problem with this model is that a ball and stick figure showing a double bond between the two carbon atoms in ethene, or any other sp2 compound for that matter, makes it appear as though two equivalent hard bonds exist between the two carbon atoms. Although the unaccounted for fourth bonding electron does in fact truly represent the second component of doubly bonded carbon atoms, the second bond is in no way equivalent to the hard sigma bond described previously. The second bond is placed on ball and stick structural diagrams purely for electron accounting purposes.
If the 4th bonding electron is not hard bound to the adjacent carbon, then how does it function as a bonding electron? This is the question that August Kekule pondered in his quest to discover the structure of benzene, and in 1886 as legend has it, discovered the answer in a dream. The fourth bonding electron, from a carbon atom in an sp2 compound, is coupled to an equivalent electron from an adjacent sp2 carbon to form what is called a pi bond. The pi bond is considered soft in that it does not form a stiff bonding field between the nuclei of the two atoms. Instead, the electron pair that make up the pi bond reside in a region above or below the nuclear plane of the carbon atom pair. Quantum chemists like to talk about the position of a bonding electron in terms of probability. That is, what is the probability of an electron being found in a certain region at some instant in time? If you apply this concept to the electrons in the pi orbital of ethene you can say, pi electrons have a very high probability of being found in a dumbbell shaped region above or below the nuclear plane of the participating carbon atoms. One can also say, pi electrons have a very low probability of being found in the inter-nuclear region of the participating carbon atoms (this region is already occupied by the sigma bonding electrons).
The volume in space occupied by the contributing pi orbital electrons from adjacent sp2 carbon atoms is then the region of influence of the second component of the doubly bonded carbon atoms in ethene or other sp2 carbon compounds. This is the real identity of the second stick used to represent the double bond in ball and stick organic structural models.
In review: The double bond of an sp2 hybridized carbon compound is composed of:
- An electron-electron sigma bond whose field of influence is between the nuclei of two adjacent carbon atoms.
- An electron-electron pi bond whose field of influence is in a region above or below the nuclear plane of the contributing carbon atoms.
The sp2 carbon atom is very important in terms of graphite because graphite is made up of almost pure sp2-type carbon atoms. The significance of sp2 carbon and graphite will be covered in more detail later. The concept of aromaticity and resonance as it relates to sp2 hybridization will be covered in another section of this site.
Sp Hybridization
Lets take this idea of bond hybridization in carbon one final step: In yet another chemical environment two carbons can triple bond to each other and use their remaining bonding electron to bond to one hydrogen atom each to form ethyne, C2H2 (acetylene). In this case carbon is bonded to only two other species. This type of bonding is known as sp hybridization. And, as in the sp2 model, the bonds indicated on a ball and stick structural diagram are used only to keep track of the total number of bonding electrons and their approximate positions.
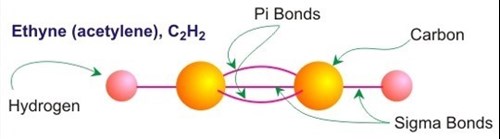
In reality there are not three equivalent bonds connecting the two carbon atoms in the ethyne molecule. There are three bonds, but only one pair of bonding electrons (one electron from each carbon atom) has a reasonable probability of being found residing between the carbon atom nuclei (sigma bond). The other 4 bonding electrons (two from each carbon) are located in two separate pi bounds. In this case the two sets of pi orbitals are offset from each other by 90. In other words, one set of pi bonds consists of two dumbbell shaped high probability electron zones located just above and below the nuclear plane of the ethyne molecule. The second two orbitals have the same shape but are inclined 90 to the first.
In summary: Ethyne (acetylene) is an sp hybridized carbon compound. In sp hybridization, carbon is bonded to two other species. The carbon-carbon bonding in sp hybridization consists of one sigma-type bond between adjacent carbon atoms, two pi bonds between adjacent carbon atoms, and one sigma type bond between carbon and hydrogen atoms.

